News
ESC Congress 2023
ANET associated events:
25 August 2023, 8:30, "Stockholm", Stroke and bleeding risk in atrial fibrillation: walking the tight rope (Paulus Kirchhof)
More information.
25 August 2023, 11:15, "Amsterdam", Hot Line Session: NOAH-AFNET 6: Oral anticoagulation in patients with atrial high rate episodes (Paulus Kirchhof)
More information.
25. August 2023, 16:30, "Hub Rembrandt", Ask the Trialist from Hot Line 1: NOAH-AFNET 6 (Paulus Kirchhof)
More information.
26 August 2023, 8:30, "Stockholm", Great Debate: controversies in atrial fibrillation - early rhythm control and atrial fibrillation burden (Andreas Götte)
More information.
28 August 2023, 08:30, "Stockholm", Novel approaches: how to provide patient-centred atrial fibrillation care (Stephan Willems)
More information.
28 August 2023, 10:15, "Hub Mondrian", Screening for atrial fibrillation: lessons learned (Renate Schnabel)
More information.
Do patients with rare and short atrial arrhythmias need anticoagulation to prevent strokes?
A review article written by the NOAH – AFNET 6 investigators led by Tobias Tönnis from UKE Hamburg summarizes recent evidence on the impact of atrial high rate episodes on stroke and cardiovascular death (1). The new evidence suggests that blood thinners may not be as effective in preventing strokes as previously thought.
Implantable devices and wearables like smart-watches enable continuous or near-continuous monitoring of cardiac rhythm. This leads to detection of short arrhythmias in many people, especially elderly persons with cardiovascular conditions. These arrhythmias, called atrial high rate episodes (AHRE), look like atrial fibrillation (AF). It is well established that blood thinners (anticoagulants) provide effective stroke prevention in patients with AF. Therefore, patients with AHRE are often treated with blood thinners as well.
Dr. Tobias Toennis, University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany, explained: “Implanted pacemakers, defibrillators, or cardiac monitors can continuously capture and quantify atrial arrhythmias. Patients with such devices are a suitable population to study the role of infrequent atrial arrhythmias for outcomes in elderly people. AHRE occur in 10-30 % of elderly patients without atrial fibrillation. We reviewed a number of previous AHRE studies and summarized the current knowledge on stroke risk.”
It remains unclear whether anticoagulants can prevent strokes in patients with AHRE. This lack of knowledge is reflected in the current guidelines worldwide. Oral anticoagulants are not routinely recommended in patients with AHRE, but decisions on anticoagulation can be individualized based on clinical risk assessment.
The reviewed trials indicate: AHRE are associated with an increased thromboembolic risk, even though it is lower than that of clinical AF. The thromboembolic risk appears to be influenced by the duration and frequency of AHRE episodes and by the number and severity of comorbidities. Recently published trials suggest that blood thinners prevent fewer strokes in patients with AHRE than previously thought.
NOAH – AFNET 6 (2), a controlled clinical trial conducted by the German Atrial Fibrillation Network (AFNET), evaluates the efficacy and safety of blood thinners in patients with AHRE. NOAH – AFNET 6 compares treatment with Edoxaban, a non-vitamin K antagonist oral anticoagulant (NOAC), to current therapy (antiplatelet therapy or no antithrombotic therapy) in patients with AHRE aged 65 years or more with at least two stroke risk factors. The trial can be expected to report soon.
The principal investigator of NOAH – AFNET 6, Prof. Kirchhof, UKE, summarized: “NOAH – AFNET 6 will provide much-needed information on the efficacy and safety of oral anticoagulation in patients with AHRE. We hope that these new data will shed some more light on the subject and that further studies will follow.”
References
(1) Toennis T et al. The influence of atrial high rate episodes on stroke and cardiovascular death – an update. Europace 22 June 2023. doi: 10.1093/europace/euad166
(2) Kirchhof et al, Probing oral anticoagulation in patients with atrial high rate episodes: Rationale and design of the Non–vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH – AFNET 6) trial, Am Heart J. 2017;190:12-18. doi: 10.1016/j.ahj.2017.04.015
About the Atrial Fibrillation NETwork (AFNET)
The Atrial Fibrillation NETwork is an interdisciplinary research network comprising scientists and physicians from hospitals and practices dedicated to improving the management of atrial fibrillation through coordinated research in Germany, Europe, and worldwide. Its main objective is to conduct high quality investigator-initiated clinical trials and registries on a national and international level. The AFNET continues the long-term activities of the network which has been funded by the German Federal Ministry of Research and Education over a decade. Since January 2015, specific projects and infrastructures of the AFNET are funded by the German Centre for Cardiovascular Research (DZHK).
Press Contact
Angelika Leute, PhD
Phone: +49 202 2623395
a.leute@t-online.de
Early rhythm control is effective and safe in AF patients irrespective of a genetic predisposition
A sub-study analysis of the EAST – AFNET 4 trial revealed how genetic risk for atrial fibrillation (AF) and stroke interacts with early rhythm control therapy: Early rhythm control reduces cardiovascular events in patients with AF across the spectrum of genetic AF and stroke risks. Today the findings were published in the journal Cardiovascular Research [1].
The EAST – AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention) trial demonstrated that rhythm control therapy – with antiarrhythmic drugs or atrial fibrillation ablation – delivered within one year after AF diagnosis improves outcomes in patients with AF and comorbidities [2]. Early rhythm control (ERC) with antiarrhythmic drugs and/or AF ablation reduced the primary outcome, a composite of cardiovascular death, stroke, and hospitalization for worsening heart failure or acute coronary syndrome, in 2789 patients with early AF and cardiovascular risk factors compared to usual care (UC) over a 5-year follow-up time.
The causes for atrial fibrillation and stroke are manifold and also comprise a heritable component. The genetic risk can be quantified by polygenic risk scores (PRS) using data from large genome-wide association studies. In a collaboration with the Broad Institute of MIT and Harvard in Cambridge, USA, these PRS were tested in the EAST – AFNET 4 study.
Dr. Shinwan Kany, University Medical Center Hamburg Eppendorf, Hamburg, Germany, explained: “Prior studies suggest that patients with a genetic predisposition to AF may suffer more recurrent AF on rhythm control therapy. Additionally, studies using PRS for stroke identified AF patients with an increase in stroke risk when otherwise classified as low risk by CHA2DS2-VASc. This information suggests that early rhythm control therapy could be less effective or less safe in patients with an elevated genomic risk for AF. To assess this, we analyzed the association between genetic AF and stroke risk and cardiovascular events in the EAST – AFNET 4 bio-sample study.”
In the EAST – AFNET 4 bio-sample sub-study, patients were asked to donate a blood sample for later analyses. For the present analysis, blood samples from 1567 of the 2789 trial patients were analyzed. Of these patients, 793 were randomized to early rhythm control, 774 to usual care. They had a median age of 71 years and comprised 44% women. Baseline characteristics were similar between randomized groups.
Consistent with the EAST – AFNET 4 main trial, early rhythm control reduced the primary outcome compared with usual care in the bio-sample sub-population (HR 0.67, p<0.001). The randomized intervention, early rhythm control, did not interact with genetic risk for AF (PRS-AF: interaction p=0.806) or stroke (PRS-Stroke: interaction p=0.765).
As expected, genetic AF risk was associated with recurrent AF, but the attributable risk was modest (HR 1.08), illustrating the effectiveness of modern rhythm control therapy across the spectrum of genetic AF risk. Unexpectedly, genetic stroke risk was associated with a higher risk for heart failure events (HR 1.23, p=0.010) without differences in stroke (HR 1.0, p=0.973) in this well-anticoagulated cohort. The association of genetic stroke risk with heart failure hospitalization was validated in the UK Biobank, a prospective cohort study of over 500,000 participants from the United Kingdom. In the replication analysis, PRS-Stroke was associated with incident AF (HR 1.16, p<0.001) and with incident heart failure (HR 1.08, p<0.001). The association with heart failure was weakened when excluding AF patients (HR 1.03, p=0.001).
EAST – AFNET 4 principal investigator, Professor Paulus Kirchhof, University Medical Center Hamburg Eppendorf, Hamburg, Germany, concluded: “Our bio-sample study shows: Early rhythm control is effective and safe across the spectrum of genetic AF and stroke risks. Our findings support the use of early rhythm control therapy irrespectively of genetic risks for AF and stroke. The association between genetic stroke risk and heart failure calls for research to understand the interactions between polygenic risks, cardiovascular diseases, and treatment.”
Since the publication of the main study result in 2020, different subgroup analyses of the EAST – AFNET 4 study data have been performed. One described the different, variable treatment patterns of antiarrhythmic drugs and AF ablation used in the trial, applied within guideline recommendations [3]. Other subgroup analyses demonstrated the prognostic benefit of early rhythm control in patients with AF and heart failure [4], in patients with asymptomatic AF [5], in patients with different AF patterns [6], in patients with high comorbidity burden [7] and in patients with prior stroke [8]. A mediator analysis identified sinus rhythm as key factor for the effectiveness of early rhythm control [9].
References
[1] Kany S, Al-Taie C, Roselli C, Pirruccello JP, Borof K, Reinbold C, Suling A, Krause L, Reissmann B, Schnabel R, Zeller T, Zapf A, Wegscheider K, Fabritz L, Ellinor PT, Kirchhof P. Association of genetic risk and outcomes in patients with early rhythm control therapy in atrial fibrillation: results from the EAST-AFNET4 study. Cardiovasc Res 2023. DOI: 10.1093/cvr/cvad027
[2] Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, Hamann F, Heidbüchel H, Hindricks G, Kautzner J, Kuck K-H, Mont L, Ng GA, Rekosz J, Schön N, Schotten U, Suling A, Taggeselle J, Themistoclakis S, Vettorazzi E, Vardas P, Wegscheider K, Willems S, Crijns HJGM, Breithardt G, for the EAST–AFNET 4 trial investigators. Early rhythm control therapy in patients with atrial fibrillation. N Engl J Med 2020; 383:1305-1316. DOI: 10.1056/NEJMoa2019422
[3] Metzner A, Suling A, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Elvan A, Goette A, Haegeli LM, Heidbuchel H, Kautzner J, Kuck KH, Mont L, Ng GA, Szumowski L, Themistoclakis S, van Gelder IC, Vardas P, Wegscheider K, Willems S, Kirchhof P. Anticoagulation, therapy of concomitant conditions, and early rhythm control therapy: a detailed analysis of treatment patterns in the EAST - AFNET 4 trial. EP Europace 2022; 24:552–564. DOI: 10.1093/europace/euab200
[4] Rillig A, Magnussen C, Ozga, Suling A, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Elvan A, Goette A, Gulizia M, Haegeli LM, Heidbuchel H, Kuck KH, Ng GA, Szumowski L, van Gelder IC, Wegscheider K, Kirchhof P. Early rhythm control therapy in patients with heart failure. Circulation 2021;144(11):845-858. DOI: 10.1161/CIRCULATIONAHA.121.056323
[5] Willems S, Borof K, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Gessler N, Goette A, Haegeli LM, Heidbuchel H, Kautzner J, Ng GA, Schnabel R, Suling A, Szumowski L, Themistoclakis S, Vardas P, van Gelder IC, Wegscheider K, Kirchhof P. Systematic, early rhythm control therapy equally improves outcomes in asymptomatic and symptomatic patients with atrial fibrillation: the EAST-AFNET 4 Trial. Eur Heart J. 2022; 43:1219-1230. DOI: 10.1093/eurheartj/ehab593.
[6] Goette a, Borof K, Breithardt G, Camm AJ, Crijns H, Kuck KH, Wegscheider K, Kirchhof P, MD. Presenting Pattern of Atrial Fibrillation and Outcomes of Early Rhythm Control Therapy. J Am Coll Cardiol. 2022; 80:283-95. DOI: 10.1016/j.jacc.2022.04.058
[7] Rillig A, Borof K, Breithardt G, Camm AJ, Crijns HJGM, Goette A, Kuck KH, Metzner A, Vardas P, Vettorazzi E, Wegscheider K, Zapf A, Kirchhof P. Early rhythm control in patients with atrial fibrillation and high comorbidity burden. Circulation. 15 Aug 2022. DOI: 10.1161/CIRCULATIONAHA.122.060274
[8] Jensen M, Suling A, Metzner A, Schnabel R, Borof K, Goette A, Haeusler KG, Zapf A, Wegscheider K, Fabritz L, Diener H-C, Thomalla G, Kirchhof P. Early rhythm-control therapy for atrial fibrillation in patients with a history of stroke: a subgroup analysis of the EAST- AFNET 4 trial. Lancet Neurol 2023; 22: 45–54. DOI: 10.1016/PIIS1474-4422(22)00436-7
[9] Eckardt L, Sehner S, Suling A, Borof K, Breithardt G, Crijns HJGM, Goette A, Wegscheider K, Zapf A, Camm AJ, Metzner A, Kirchhof P. Attaining sinus rhythm mediates improved outcome with early rhythm control therapy of atrial fibrillation: the EAST – AFNET 4 trial. Eur Heart J, 2022. DOI: 10.1093/eurheartj/ehac471
[10] Gottschalk S, Kany S, König H-H, Crijns HJGM, Vardas P, Camm AJ, Wegscheider K, Metzner A, Rillig A, Kirchhof P, Dams J. Cost- effectiveness of early rhythm-control versus usual care in atrial fibrillation care: an analysis based on the German subsample of the EAST-AFNET 4 trial. EP Europace 2023. DOI: 10.1093/europace/euad051
Press Contact
Angelika Leute, PhD
Phone: +49 202 2623395
a.leute@t-online.de
Follow us on Twitter @afnet_ev and hashtag #EASTtrial.
Funding: AFNET, BMBF, DZHK, EHRA, Deutsche Herzstiftung, Abbott, Sanofi
About the EAST – AFNET 4 trial
EAST – AFNET 4 is an investigator-initiated trial (IIT) that compared two different treatment strategies in atrial fibrillation. The EAST – AFNET 4 trial tested whether an early, comprehensive rhythm control therapy can prevent adverse cardiovascular outcomes in patients with atrial fibrillation (AF) compared to usual care.
A total of 2789 patients with early AF (diagnosed less than a year ago) and at least two cardiovascular conditions (approximating a CHA₂DS₂-VASc score >=2) were enrolled by 135 sites in 11 countries during 2011 to 2016. Patients were randomized 1:1 to early rhythm control therapy or usual care, stratified by sites. Patients in both groups received guideline-recommended treatment for underlying cardiovascular conditions, anticoagulation, and rate control.
All patients in the early rhythm control group received antiarrhythmic drugs or catheter ablation after randomization (chosen by the local study teams). Rhythm control therapy was escalated with AF ablation and/or antiarrhythmic drugs when recurrent AF was documented clinically or by ECG, including monitoring with patient-operated ECG devices.
Patients in the usual care group were initially managed with rate control. Rhythm control therapy was only used to improve atrial fibrillation-related symptoms despite optimal rate control, following current guidelines.
About the Atrial Fibrillation NETwork (AFNET)
The Atrial Fibrillation NETwork is an interdisciplinary research network comprising scientists and physicians from hospitals and practices dedicated to improving the management of atrial fibrillation through coordinated research in Germany, Europe, and worldwide. Its main objective is to conduct high quality investigator-initiated clinical trials and registries on a national and international level. The AFNET continues the long-term activities of the network which has been funded by the German Federal Ministry of Research and Education over a decade. Since January 2015, specific projects and infrastructures of the AFNET are funded by the German Centre for Cardiovascular Research (DZHK).
www.af-net.eu
Covid-19 pandemic traumatized physicians working in hospitals and private practices
The Covid-19 pandemic entailed exceptional circumstances posing major challenges to the majority of people. Here, health care workers were particularly concerned. A relevant question was: Were physicians able to overcome these extreme stress situations in their professional lives? Or was their mental health afflicted? To approach these issues Prof. Andreas Goette, cardiologist at St. Vincenz-Krankenhaus Paderborn, Germany, and Prof. Karl-Heinz Ladwig, specialist in psychosomatic medicine at Technische Universität München (TUM), Germany, surveyed physicians during the pandemic and focused not only on those working in hospitals, but for the first time also on physicians in private practices.
How did physicians experience their own medical care during the pandemic? How did they handle extreme burdening situations? Physicians of different specializations expressed their appraisal in an anonymized online survey in autumn 2021. The study was conducted in Germany in cooperation with the academic research organization Atrial Fibrillation NETwork (AFNET) in Münster and the Medical Council of the German district Westfalen-Lippe. The results were published in April 2023.
“In the second year of the pandemic, reporting about overstressed and exhausted physicians increased. in order to investigate the problem by scientific methods. we conducted this systematic study.” Prof. Goette explained. “During data analysis we were also interested in the following questions: How do the adverse effects of the pandemic differ in physicians working in hospitals compared to those working in private practices? Is long-term professional experience helpful to cope with these stresses? Are there sex-related differences regarding the effect of the pandemic on medical care?”
1476 physicians in North Rhine Westphalia, Germany, participated in the online survey conducted over a period of six weeks at the end of the year 2021. Items covered the physicians` life situation, the characteristics of their patients, and the threats the physicians were exposed to.
1139 of the participants reported personal treatment experiences with Covid-19 patients. Of these, 553 physicians worked in a private practice and 586 in a hospital. They were general practitioners or specialists in internal medicine, surgery, gynecology, or pediatrics.
Covid-19 provoked profound conflicts between professional and ethical values. More than one third of the physicians, especially those working in private practices, felt restrained in their medical care by external constraints. Nearly half of the hospital physicians (48%) and more than one forth of the physicians working in private practices (27%) reported cases in which they experienced profound difficulties to maintain the dignity of their patients during the pandemic.
Of note, on the peak of the fourth wave of the pandemic, one fourth (23%) of the physicians under investigation suffered from clinical meaningful depressive symptoms and another forth (24%) from anxiety. Comparison with studies during the early phase of the pandemic suggest an increase of affective burden.
More than half of the physicians expressed feelings of helplessness (63% in hospitals and 53% in private practices). The majority complained of sleeping problems. Women and physicians with minor years of medical experience were particularly affected.
Prof. Ladwig summarized: “The results of our study reveal: The pandemic and especially the treatment of Covid-19 patients had serious consequences for medical care in hospitals and private practices. To some extent, medical care was impugned in its ethical features. The traumatizing work content did not leave physicians untouched but induced mental health distortions even in experienced doctors who are rather used to manage difficult situations. As we see, physicians were not able to adapt to the adverse situation in the course of the pandemic but in contrast emotional burden increased. Emotional perturbations among physicians have attained a critical magnitude.”
Prof. Goette concluded: “It is depressing to see, how the mental burden of us physicians increased steadily over the pandemic. The rate of substantial psychic impact of the fourth Covid-19 wave appears really significant. The end of the pandemic will hopefully cause recovery of the findings. However, this remains to be seen and requires further research.”
Publication
Ladwig KH et al. Covid‑19 pandemic induced traumatizing medical job contents and mental health distortions of physicians working in private practices and in hospitals. Nature Scientific Reports. 2023; 13:5284. DOI: 10.1038/s41598-023-32412-y
About the Atrial Fibrillation NETwork (AFNET)
The Atrial Fibrillation NETwork is an interdisciplinary research network comprising scientists and physicians from hospitals and practices dedicated to improving the management of atrial fibrillation through coordinated research in Germany, Europe, and worldwide. Its main objective is to conduct high quality investigator-initiated clinical trials and registries on a national and international level. The AFNET continues the long-term activities of the network which has been funded by the German Federal Ministry of Research and Education over a decade. Since January 2015, specific projects and infrastructures of the AFNET are funded by the German Centre for Cardiovascular Research (DZHK).
www.af-net.eu
Press contact
Dr. Angelika Leute
Tel: 0202 2623395
a.leute@t-online.de
Is early rhythm control in atrial fibrillation care cost-effective? An analysis based on the EAST – AFNET 4 trial
Press release
Patients with atrial fibrillation (AF) benefit from early rhythm control therapy. It reduces cardiovascular deaths, strokes, and other adverse outcomes by 20% compared to usual care. The beneficial effects of early rhythm control were shown by the pan-European EAST – AFNET 4 trial and confirmed by other large health studies. However, what is the price of the new treatment strategy? A cost-effectiveness analysis revealed: the health benefits of early rhythm control come at reasonable additional costs. The analysis was published today in EP Europace, a journal of the European Society of Cardiology (ESC) [1].
Atrial fibrillation is a rising epidemic. The number of AF patients in the European Union is projected to increase to approximately 18 million by 2060. Affected persons are at higher risk for stroke and other adverse events associated with high costs for treatment and long-term care. This leads to an increasing economic burden.
The EAST – AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention) trial investigated whether rhythm control therapy – with antiarrhythmic drugs or atrial fibrillation ablation – delivered within one year after AF diagnosis improves outcomes. The main study result, published in 2020 [2], demonstrated that early rhythm control therapy improves outcomes in patients with AF and comorbidities: early rhythm control with antiarrhythmic drugs and/or AF ablation reduced the primary outcome, a composite of cardiovascular death, stroke, and hospitalisation for worsening heart failure or acute coronary syndrome, in 2,789 patients with early AF and cardiovascular risk factors compared to usual care over a 5-year follow-up.
“The health benefit of early rhythm control is evident. However, the cost-effectiveness of the new treatment strategy has not been evaluated so far. One concern after the publication of the EAST – AFNET 4 main study was whether the additional treatment would add an acceptable or undue financial burden to healthcare systems. In the current analysis, we examined for the first time the cost-effectiveness of early rhythm control compared to usual care,” explained Sophie Gottschalk, Department of Health Economics and Health Services Research, University Medical Center Hamburg-Eppendorf, Hamburg Center for Health Economics, Hamburg, Germany.
The cost-effectiveness analysis was based on data from the German subsample of the EAST – AFNET 4 trial comprising 1,664 patients (see graphical abstract). Early rhythm control in 832 patients was compared to usual care in 832 patients over a 6-year time horizon and from a German healthcare payer perspective. Cost categories considered in the analysis were hospitalisation and medication costs. The time to the occurrence of a primary outcome and the time survived in the observation period were used as effect measures.
Incremental cost-effectiveness ratios (ICERs) were calculated for each effect measure. The ICER value describes the additional costs needed for an additional year free of primary outcome events or an additional life year, respectively. The analysis showed: early rhythm control was associated with higher costs to the amount of €1,924, resulting in ICERs of €10,638 per additional year without a primary outcome, and €22,536 per life year gained. As shown in the uncertainty analysis, high probabilities of cost-effectiveness could be reached if the healthcare payer was willing to pay ≥€55,000 for an additional year without a primary outcome or for a life year gained (probabilities ≥95% or ≥80%, respectively).
The principal investigator of EAST – AFNET 4, Prof. Paulus Kirchhof, Department of Cardiology, Universit Heart and Vascular Center Hamburg-Eppendorf, Hamburg, Germany, concluded: "The health benefits of early rhythm control come at reasonable additional costs as indicated by our analysis of the EAST – AFNET 4 study data for Germany. The added cost is comparable to the cost of other therapies reducing outcomes, and within typical ranges of acceptable payments in Germany. Future studies examining the cost-effectiveness of early rhythm control in other countries, in subgroups with higher benefit from rhythm control therapy, or the cost-effectiveness of different modes of early rhythm control are warranted.”
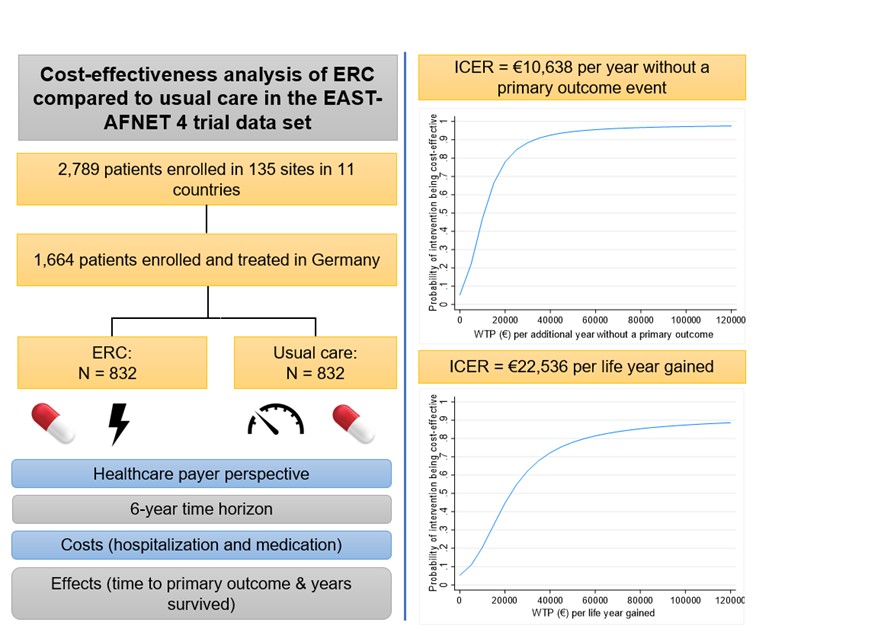
Notes: ERC, early rhythm control therapy; ICER, incremental cost-effectiveness ratio; primary outcome event = cardiovascular death, stroke, or hospitalisation for stroke or acute coronary syndrome.
References
[1] Gottschalk S, Kany S, König H-H, Crijns HJGM, Vardas P, Camm AJ, Wegscheider K, Metzner A, Rillig A, Kirchhof P, Dams J. Cost- effectiveness of early rhythm-control versus usual care in atrial fibrillation care: an analysis based on the German subsample of the EAST-AFNET 4 trial. EP Europace 2023. DOI: 10.1093/europace/euad051
[2] Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, Hamann F, Heidbüchel H, Hindricks G, Kautzner J, Kuck K-H, Mont L, Ng GA, Rekosz J, Schön N, Schotten U, Suling A, Taggeselle J, Themistoclakis S, Vettorazzi E, Vardas P, Wegscheider K, Willems S, Crijns HJGM, Breithardt G, for the EAST–AFNET 4 trial investigators. Early rhythm control therapy in patients with atrial fibrillation. N Engl J Med 2020; 383:1305-1316. DOI: 10.1056/NEJMoa2019422
[3] Metzner A, Suling A, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Elvan A, Goette A, Haegeli LM, Heidbuchel H, Kautzner J, Kuck KH, Mont L, Ng GA, Szumowski L, Themistoclakis S, van Gelder IC, Vardas P, Wegscheider K, Willems S, Kirchhof P. Anticoagulation, therapy of concomitant conditions, and early rhythm control therapy: a detailed analysis of treatment patterns in the EAST - AFNET 4 trial. EP Europace 2022; 24:552–564. DOI: 10.1093/europace/euab200
[4] Rillig A, Magnussen C, Ozga, Suling A, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Elvan A, Goette A, Gulizia M, Haegeli LM, Heidbuchel H, Kuck KH, Ng GA, Szumowski L, van Gelder IC, Wegscheider K, Kirchhof P. Early rhythm control therapy in patients with heart failure. Circulation 2021;144(11):845-858. DOI: 10.1161/CIRCULATIONAHA.121.056323
[5] Willems S, Borof K, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Gessler N, Goette A, Haegeli LM, Heidbuchel H, Kautzner J, Ng GA, Schnabel R, Suling A, Szumowski L, Themistoclakis S, Vardas P, van Gelder IC, Wegscheider K, Kirchhof P. Systematic, early rhythm control therapy equally improves outcomes in asymptomatic and symptomatic patients with atrial fibrillation: the EAST-AFNET 4 Trial. Eur Heart J. 2022; 43:1219-1230. DOI: 10.1093/eurheartj/ehab593
[6] Goette a, Borof K, Breithardt G, Camm AJ, Crijns H, Kuck KH, Wegscheider K, Kirchhof P, MD. Presenting Pattern of Atrial Fibrillation and Outcomes of Early Rhythm Control Therapy. J Am Coll Cardiol. 2022; 80:283-95. DOI: 10.1016/j.jacc.2022.04.058
[7] Rillig A, Borof K, Breithardt G, Camm AJ, Crijns HJGM, Goette A, Kuck KH, Metzner A, Vardas P, Vettorazzi E, Wegscheider K, Zapf A, Kirchhof P. Early rhythm control in patients with atrial fibrillation and high comorbidity burden. Circulation. 15 Aug 2022. DOI: 10.1161/CIRCULATIONAHA.122.060274
[8] Jensen M, Suling A, Metzner A, Schnabel R, Borof K, Goette A, Haeusler KG, Zapf A, Wegscheider K, Fabritz L, Diener H-C, Thomalla G, Kirchhof P. Early rhythm-control therapy for atrial fibrillation in patients with a history of stroke: a subgroup analysis of the EAST- AFNET 4 trial. Lancet Neurol 2023; 22: 45–54. DOI: 10.1016/PIIS1474-4422(22)00436-7
[9] Eckardt L, Sehner S, Suling A, Borof K, Breithardt G, Crijns HJGM, Goette A, Wegscheider K, Zapf A, Camm AJ, Metzner A, Kirchhof P. Attaining sinus rhythm mediates improved outcome with early rhythm control therapy of atrial fibrillation: the EAST – AFNET 4 trial. Eur Heart J, 2022. DOI: 10.1093/eurheartj/ehac471
Twitter: @afnet_ev, hashtag #EASTtrial.
Funding: AFNET, BMBF, DZHK, EHRA, Deutsche Herzstiftung, Abbott, Sanofi.
About the EAST – AFNET 4 trial
EAST – AFNET 4 is an investigator-initiated trial (IIT) that compared two different treatment strategies in atrial fibrillation. The EAST – AFNET 4 trial tested whether an early, comprehensive rhythm control therapy can prevent adverse cardiovascular outcomes in patients with atrial fibrillation (AF) compared to usual care.
A total of 2,789 patients with early AF (diagnosed less than a year ago) and at least two cardiovascular conditions (approximating a CHA₂DS₂-VASc score >=2) were enrolled by 135 sites in 11 countries during 2011 to 2016. Patients were randomised 1:1 to early rhythm control therapy or usual care, stratified by sites. Patients in both groups received guideline-recommended treatment for underlying cardiovascular conditions, anticoagulation, and rate control.
All patients in the early rhythm control group received antiarrhythmic drugs or catheter ablation after randomisation (chosen by the local study teams). Rhythm control therapy was escalated with AF ablation and/or antiarrhythmic drugs when recurrent AF was documented clinically or by ECG, including monitoring with patient-operated ECG devices.
Patients in the usual care group were initially managed with rate control. Rhythm control therapy was only used to improve atrial fibrillation-related symptoms despite optimal rate control, following current guidelines.
About the Atrial Fibrillation NETwork (AFNET)
The Atrial Fibrillation NETwork is an interdisciplinary research network comprising scientists and physicians from hospitals and practices dedicated to improving the management of atrial fibrillation through coordinated research in Germany, Europe, and worldwide. Its main objective is to conduct high quality investigator-initiated clinical trials and registries on a national and international level. The AFNET continues the long-term activities of the network which has been funded by the German Federal Ministry of Research and Education over a decade. Since January 2015, specific projects and infrastructures of the AFNET are funded by the German Centre for Cardiovascular Research (DZHK).
Press contact
Angelika Leute, PhD
Phone: +49 202 2623395
