AFNET was founded in 2003 as one of the 21 research networks in Germany funded by the German Federal Ministry of Research and Education (BMBF). Over the years, the organisation has developed into an internationally active academic research organisation dedicated to translational cardiovascular research and evaluating new therapies mainly focusing on atrial fibrillation. A description of the development of AFNET from Translational Research Network into an Academic Research Organisation was published in 2016 in the German journal “Bundesgesundheitsblatt”.
Membership is possible for physicians, scientists, hospitals, private practices, and companies.
AFNET – an academic research organisation committed to improving the lives of patients with cardiovascular diseases.
The mission of AFNET is to improve the lives of patients with cardiovascular diseases by generating knowledge on their mechanisms and evidence on effective and safe treatments. To achieve this, AFNET provides a platform to plan and execute non-commercial, international, investigator-initiated controlled clinical trials, registries, and translational research projects that can inform better care of patients with cardiovascular disease. AFNET cooperates with selected partners to conduct its projects. Funding is obtained through public and/or private partnerships. AFNET has long expertise in the management of atrial fibrillation, but also provides support for research in other fields informing cardiovascular care.
Until 2013, AFNET was integrated into the University Hospital Münster in Germany. The growing scientific activities of AFNET called for an adjustment of its structure. Therefore, AFNET was registered as an association in Germany (e.V. = “eingetragener Verein”) in 2013 that acts as an independent, not-for-profit organisation. This structure enables sponsorship roles for international clinical trials and participation in international research consortia. AFNET is led by a Board of Directors (Andreas Goette, Paulus Kirchhof, Ulrich Schotten, Stephan Willems). The board is advised by a Steering Committee consisting of fifteen cardiologists, statisticians, and cardiovascular researchers. An Advisory Board consisting of our past chairperson, Günter Breithardt, a legal expert, and a health care manager, oversees the work. All committee members work on an honorary basis. Their main employers recognise the scientific and public value of their work for AFNET. The AFNET team in the central office is located in Münster in an innovation campus at the University of Münster and consists of 12 individuals mainly focussing on project management for clinical studies and scientific communication support. AFNET staff are paid according to German university salary scales.
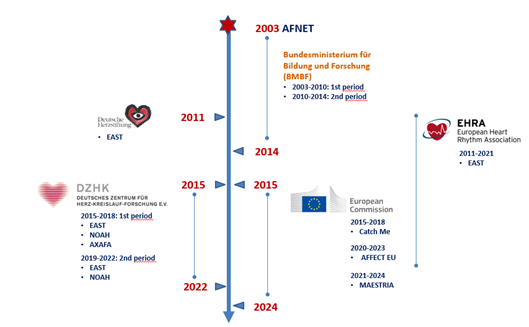
AFNET was initially fully funded by the BMBF. The association receives some core funding from the German Centre for Cardiovascular Research (DZHK). Its main funding comes from research projects, typically funded in public-private partnership, and EU grants supporting data science in its clinical data sets. AFNET has received public funding from different sources on national and international level in Europe throughout the last 20 years.
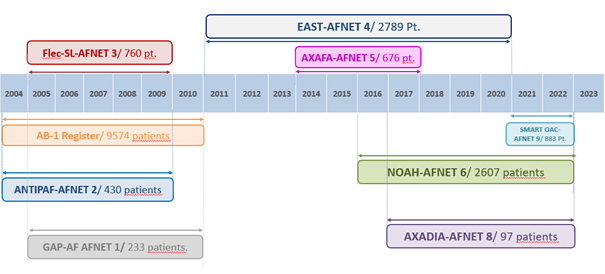
AFNET trials. So far, AFNET successfully delivered eight investigator-initiated controlled clinical trials. The first three trials (GAPAF-AFNET 1 (NCT00293943), ANTIPAF-AFNET 2 (NCT00098137), Flec SL-AFNET 3 (NCT00215774)) were conducted in Germany and funded by the BMBF with additional support from industry partners. Legal sponsor for these trials were different universities in Germany. For the later trials, AFNET association served as legal sponsor. Five trials (EAST-AFNET 4 (NCT01288352), AXAFA-AFNET 5 (NCT02227550), NOAH-AFNET 6 (NCT02618577), AXADIA-AFNET8 (NCT02933697), Smart in OAC-AFNET 9 (NCT04579159)) were successfully conducted to time and target in Europe and the USA. AXAFA-AFNET 5 reported in 2018 and informed the use of factor Xa antagonists around AF ablation. EAST-AFNET 4 reported its practice-changing findings on early rhythm control in 2020. AXADIA –AFNET 8 evaluated different types of anticaogulation in patients with atrial fibrillation on hemodialysis. The non-randomized Smart in OAC-AFNET 9 trial developed a fully digital enrolment and follow-up procedure incorporating PPG-based detection of atrial arrhythmias. NOAH-AFNET 6 will report in August 2023. AFNET delivered these trials in up to 18 countries in Europe and North America with nimble, clinician-led management. The academic ownership and AFNET´ s role as legal sponsor enabled further use of the data sets for sub analyses and data science.
AFNET/EHRA consensus conferences. AFNET coordinates a series of consensus conferences with the European Heart Rhythm Association. The proceedings of these meetings are published in consensus papers that informed the work of researchers, research funders, and the European Medicines Agency.
You can download the articles of association of the Atrial Fibrillation NETwork (Kompetenznetz Vorhofflimmern e.V.) here.
Partner in scientific consortia. AFNET provides its expertise in clinical trials to scientific partners who desire support for their clinical trials, e.g. by supporting trials funded by the German Center of Cardiovascular Research (DZHK). AFNET has been a partner and work package leader in several consortia funded by the horizon 2020 programme of the European Union, including CATCH ME, BigData@Heart, and MAESTRIA.
Partner in clinical trials. AFNET is also a partner in a number of ongoing controlled clinical trials conducted by the German Centre for Cardiovascular Research (DZHK) and other sponsors, including CMR-ICD (NCT04558723), CABA-HFPEF (NCT05508256), CLOSURE-AF (NCT03463317), and OCEAN (NCT02168829). AFNET provides regulatory project management for these trials and is assisting with other tasks such as organizing investigator meetings and writing study documents such as newsletters, charters, and patient information/informed consent.
AFNET is open for new research cooperations. Institutions, companies and scientists are welcome to approach us with research ideas and project proposals. If you are interested in a cooperation with AFNET, please contact us at info@kompetenznetz-vorhofflimmern.de.
Below you find some of the institutions AFNET is a current cooperation with. The supporter of each study and project are listed on the respective study site.




(Link ist extern)(Link ist extern)(Link ist extern)(Link ist extern)European Society of Cardiology (ESC)

(Link ist extern)(Link ist extern)(Link ist extern)(Link ist extern)European Heart Rhythm Association (EHRA)

(Link ist extern)(Link ist extern)(Link ist extern)(Link ist extern)Bundesverband Niedergelassener Kardiologe (BNK)

(Link ist extern)(Link ist extern)(Link ist extern)(Link ist extern)(Link ist extern)Kompetenznetz Angeborene Herzfehler (KNAHF)

(Link ist extern)(Link ist extern)(Link ist extern)(Link ist extern)(Link ist extern)(Link ist extern)Kompetenznetz Herzinsuffizienz (KNHI)

Visit our sustainability profile on Integrity Next which shows our compliance regarding:
- Quality management
- Data protecion (GDPR)
- Conflict of interest
- Anti-corruption and anti-bribery
- Occupational safety
- Covid-19 assessment
- Management
- Diversity and inclusion
You can download the gender equality plan of the Atrial Fibrillation NETwork (Kompetenznetz Vorhofflimmern e.V.) here.

